Dutta Lab
Tonix Pharmaceuticals announces plan to develop Dutta Lab technology for PTSD and other disorders
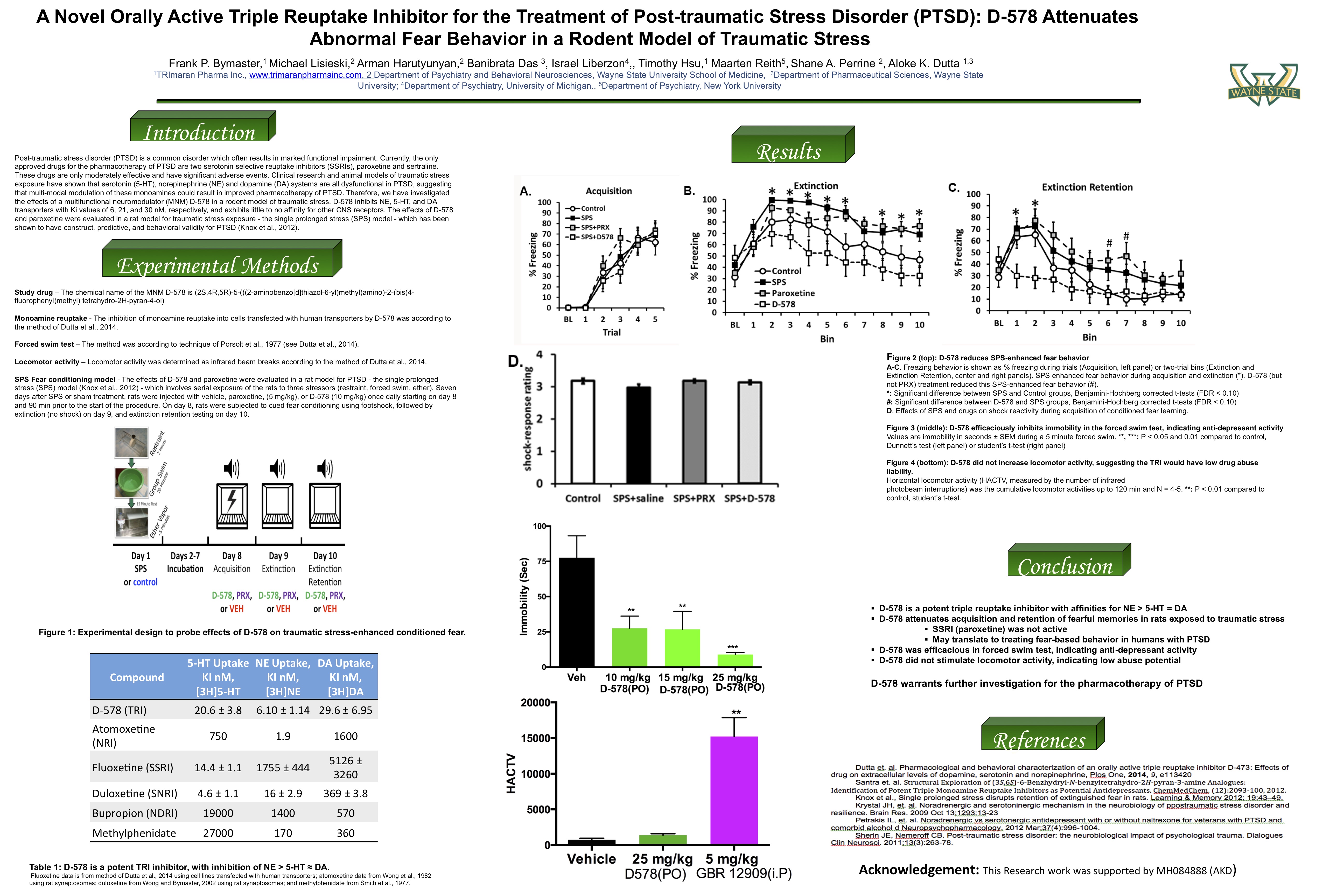
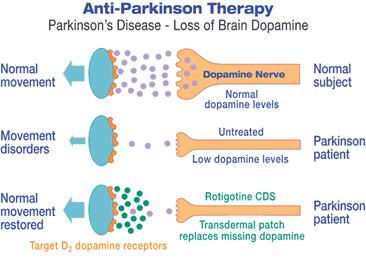
Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (Tonix or the Company), a clinical-stage biopharmaceutical company, announced on Aug. 20, 2019, an exclusive agreement to in-license a triple reuptake inhibitor (TRI), TNX-1600 (formerly D-578), to treat posttraumatic stress disorder (PTSD) and potentially other central nervous system (CNS) disorders. The compound was developed and pharmacologically characterized by Aloke Dutta, Ph.D., Professor of Pharmaceutical Sciences at Wayne State University, with funding from a National Institutes of Health grant (grant number MH084888), and the patents covering the compounds were licensed to TRImaran Pharma, Inc. (TRImaran).
Dr. Dutta presented pharmacological and behavioral efficacy of the compound in PTSD and depression animal models at the MHSRS conference in Orlando, FL, in August 2019 (poster below).
More research | |
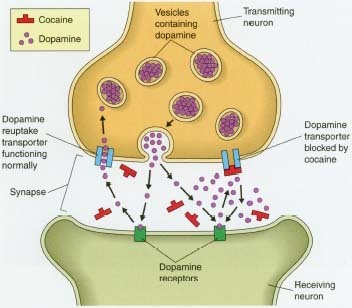 | |
 |  |